

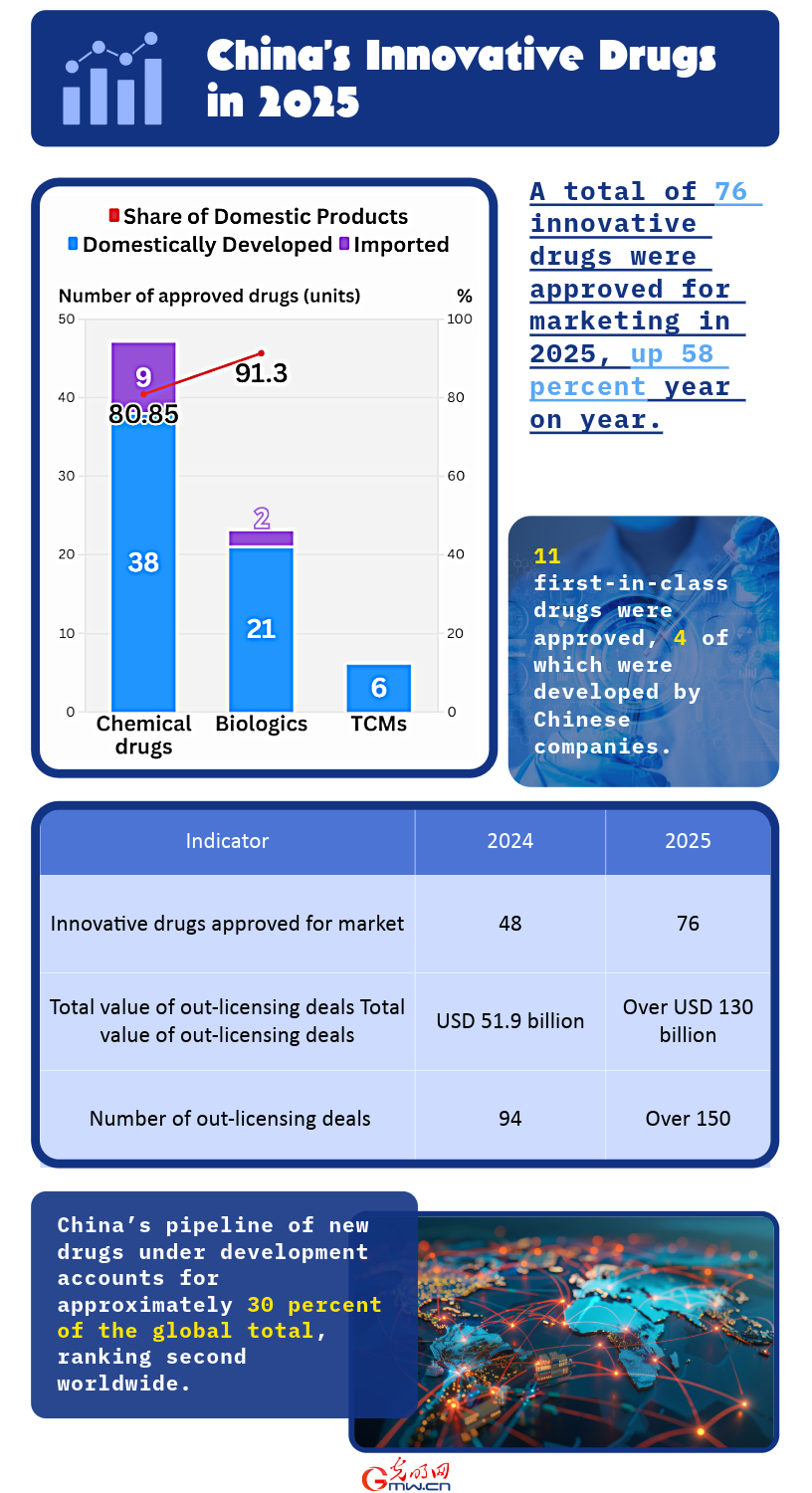
BEIJING — China approved a total of 76 innovative drugs for market launch in 2025, a sharp increase from 48 in 2024 and a record high, according to the National Medical Products Administration (NMPA).
The approved products included 47 chemical drugs, 23 biologics and six traditional Chinese medicines (TCMs). Eighty-one percent of the chemical drugs (38 products) and 91.30 percent of the biologic products (21 products) were developed by Chinese pharmaceutical companies.
Eleven of the approved drugs were First-in-Class medicines featuring novel therapeutic mechanisms, four of which were independently developed by Chinese firms.
The NMPA also said that the total value of China's outbound innovative drug licensing transactions exceeded 130 billion USD in 2025, with more than 150 licensing deals signed, far surpassing the 51.9 billion USD across 94 deals recorded in 2024, both setting new records. China's pipeline of new drugs under development accounts for approximately 30 percent of the global total, ranking second worldwide.

Various festive events held across China to celebrate upcoming Chinese New Year


Hit epic drama sparks interest in lesser-studied chapter of ancient Chinese history

Lanterns hoisted to mark upcoming Chinese New Year in Hong Kong

"In-train fair" launched in NE China's Heilongjiang amid Spring Festival travel rush
点击右上角![]() 微信好友
微信好友
 朋友圈
朋友圈
请使用浏览器分享功能进行分享
