Mass inoculation shows safety, efficacy of Chinese COVID-19 vaccines: expert

Chinese respiratory specialist Zhong Nanshan speaks in an interview in Guangzhou, south China's Guangdong Province, July 29, 2020. (Xinhua/Liu Dawei)
GUANGZHOU, Jan. 31 (Xinhua) -- China's renowned respiratory-disease expert Zhong Nanshan said Sunday that the mass inoculation of homegrown COVID-19 vaccines underway in China shows the vaccines are safe and effective.
The two vaccines currently in use in China, the China National Biotec Group (CNBG) COVID-19 vaccine and the CoronaVac vaccine developed by China's Sinovac Biotech Ltd., are both inactivated vaccines that are relatively safe, Zhong said at the launch ceremony of an event held in south China's Guangdong Province to promote the use of technology in COVID-19 prevention and control.
According to the Chinese Center for Disease Control and Prevention (China CDC), more than 24 million doses of COVID-19 vaccines had been administered in China by Sunday.
"The rate of the vaccines' mild adverse reactions, which include fever, soar arms and other symptoms, is six per 100,000 people," Zhong said.
The rate of severe adverse vaccine reactions is one in a million, only one third of that of flu vaccines, he said.
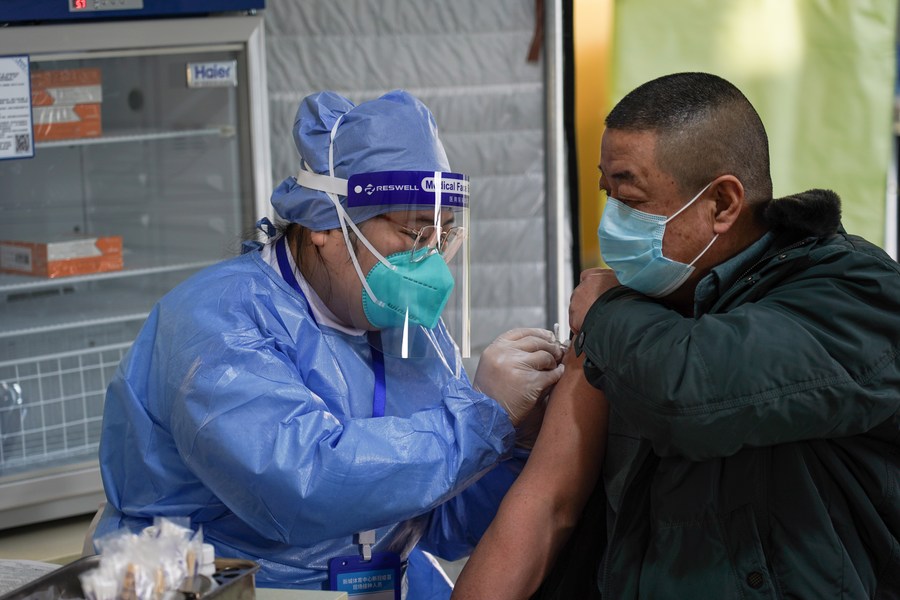
A resident receives the second dose of the COVID-19 vaccine at a sports center in Daxing District, Beijing, capital of China, Jan. 23, 2021. (Xinhua/Peng Ziyang)
On the same day, Xu Wenbo, head of the National Institute for Viral Disease Control and Prevention under the China CDC, also said the incidence of severe abnormal reactions caused by the COVID-19 vaccines currently used in China is no higher than that of the influenza vaccines.
In terms of the Chinese vaccines' efficacy, Zhong said the vaccines can protect people against COVID-19 for at least six months.
"We found that antibody levels in the first batch of inoculated people in China remained at 90 percent of their original levels after nearly eight months," he said, adding that the Chinese vaccines' effective period has yet to be confirmed with precision.
The difference in the vaccines' efficacy rates shown by clinical trials in various countries is due to varying standards used in those countries, he said.
"The phase-1 and phase-2 clinical trials of the two Chinese vaccines were conducted domestically, while the phase-3 trials were carried out in various countries because there were too few COVID-19 cases in China then," he said. "The countries apply different standards. In some countries, most of the vaccinated people are medical workers who have very high exposure risks."
[ Editor: WXY ]




More From Guangming Online
Medics from Fujian leave for Shanghai to aid in battle against COVID-19 resurgence
New int'l land-sea transport service to Indo-China Peninsula launched
Another makeshift hospital under construction in Shanghai
Tourists view tulips in Suiping County, Henan
In pics: blooming gagea flowers on grassland in Zhaosu, Xinjiang
Greek workers stage 24-hour general strike over high prices